Research
The Day lab studies G quadruplex (G4) DNA, a non-canonical DNA structure formed from stacks of planar tetrads held together by hydrogen-bonded guanines. If the DNA helix resembles “two helical chains each coiled round the same axis,” (Watson and Crick, 1953) then G4 DNA resembles a knot in one of those chains. Quadruplexes are found in genomes ranging from prokaryotes to plants to mammals and an estimated ~10,000 G4 structures are found in the human genome in a non-random distribution. G4 DNA is enriched gene regulatory regions and telomeric sequences.
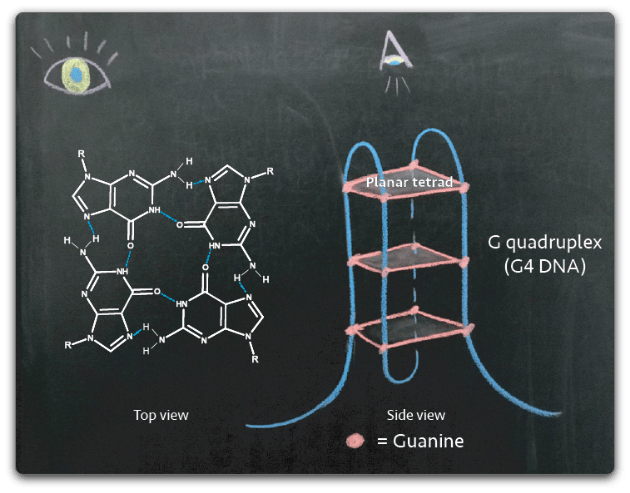
G4 DNA plays a physiological role in regulation of transcription and initiation of DNA replication. However, the same structures pose a threat to genome stability; these ‘knots’ in the DNA can prove difficult for a polymerase to copy and frequently lead to stalled replication forks followed by breaks in the DNA molecule. Once a DNA break has occurred, the presence of G4 DNA near the break can make the lesion more difficult to repair as cellular machinery processes G4 DNA inefficiently. Therefore, the need to control G4 DNA stems from a need to limit genomic instability.
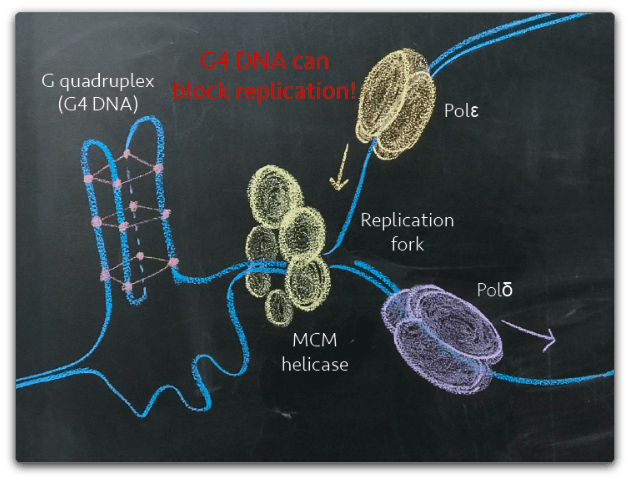
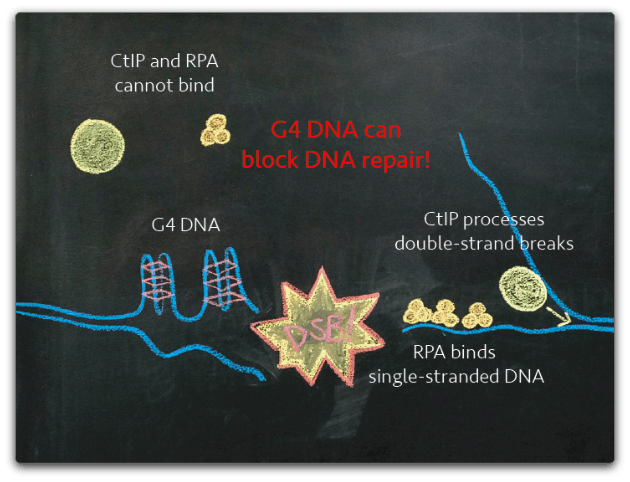
How do cells control G4 DNA?
Naked DNA forms ~70-fold more G4 structures than nucleosome-bound DNA, suggesting that the chromatin environment exerts influence over the formation of G4 DNA in vivo. Further, we hypothesize that specific histone modifications promote or suppress the formation of G4 DNA in the nucleus. Our lab investigates epigenetic mechanisms of G4 DNA regulation. These studies will help us to understand how cells control G4 DNA for physiological functions and suppress it to limit genomic instability that contributes to mutagenesis and human disease.
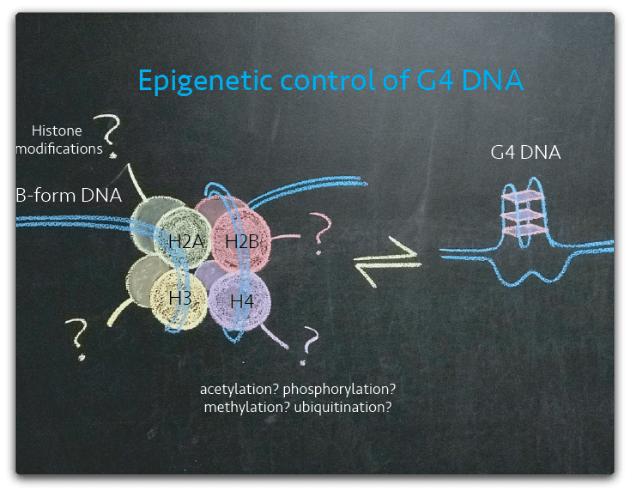
How does G4 DNA control cells ?
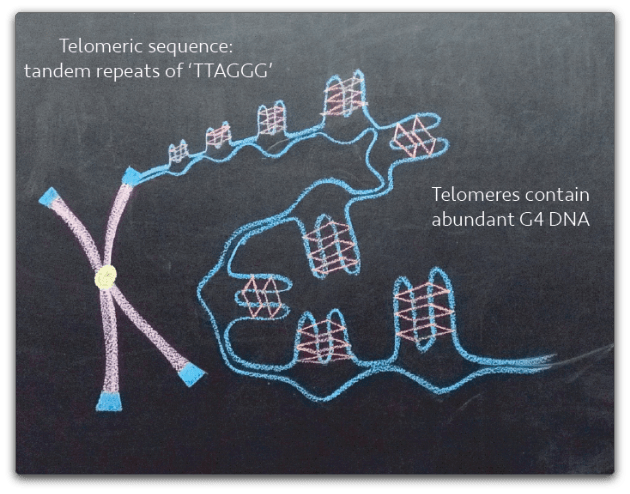
Our lab is also focused on the role of G4 DNA at mammalian telomeres, repetitive, guanine-rich sequences. Telomeres protect chromosome ends from degradation and contain abundant G4 DNA structures. In mammalian cells, failure to maintain appropriate telomere integrity and length has been linked to both cancer and aging. We study the mechanistic relationship between the accumulation of G4 DNA and activation of telomere maintenance pathways. The goal of these studies is to identify therapeutic targets to treat pathogenic conditions resulting from telomere dysregulation.
